Oxidative processes
These processes chemically modify the micropollutants following the addition of an oxidising agent. The micropollutants are oxidised, which results in the micropollutants losing their original properties. The aim of these processes is to chemically break down the micropollutants to the greatest possible extent in order to minimise their environmental impact.
However, complete mineralisation of the substances is often difficult to achieve. In most cases, the micropollutants are merely transformed into different oxidation products. These transformation products can be problematic for compliance with the BOD5 threshold value, since they generally have favourable properties regarding biodegradability and can thus increase the effluent value.[1] Furthermore, their environmental behaviour and toxicity have often not been fully investigated. According to the current status of science, it is therefore recommended that wastewater that has been treated in an oxidative process be re-treated in a biologically active stage in order to maximise the removal of both the residual oxidants and the newly formed substances from the treated wastewater.
In addition to ozonation, the oxidative processes also include the ‘Advanced Oxidation Processes’ (AOP). According to current knowledge, ozonation is regarded as a suitable and effective process for large-scale implementation. While the AOPs are generally capable of removing a wide range of micropollutants from the wastewater, they consume a considerably higher level of energy, however, resulting in higher costs than ozonation. Therefore, the AOPs are classified as unsuitable for use in municipal wastewater treatment.
-
> Ozone
Pulse CMS Ozone is a powerful oxidising agent that reacts in aqueous media in two ways [1]:
- Selective, direct reaction with water constituents which have electron-rich bonds (e.g. amino groups, double bonds and activating aromatic groups).
- Radical chain reaction by cleavage of the ozone into hydroxyl radicals, which attack various types of water constituents very quickly and unspecifically.
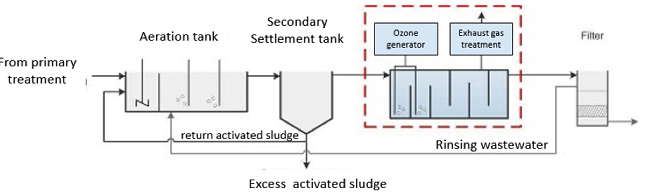
Ozone reacts not only with the micropollutants to be eliminated, but also with other wastewater constituents such as the DOC, solids or nitrite. In order to minimise the ozone consumption, use of ozone is recommended after the biological treatment. In the recent large-scale projects, ozonation has been arranged for the treatment of biologically treated wastewater after the secondary sedimentation stage. The following components are necessary for the technical implementation of an ozone treatment stage in municipal wastewater treatment plants [2]:
- Ozone generator: The ozone generator produces the required ozone from oxygen or dry air on site. Ozone production is energy-intensive and generates heat, which requires cooling.
- Contact reactor: In the contact reactor, the ozone is introduced into the wastewater. The ozone reacts with the micropollutants and other wastewater constituents. In order to achieve a high elimination performance and efficient use of the ozone, the retention time in the reactor must be sufficiently long. This can be achieved by cascading the reactor, for example. The reactor must be sealed in a gas-tight manner to protect the operating personnel from escaping ozone.
- Exhaust gas treatment: This component serves to destroy the residual ozone in the exhaust air of the reactor.
- After-treatment: To remove the developing transformation products, it is recommended that a biologically active process (e.g. sand filter, maturation pond) or an activated carbon filter be installed downstream.
- Process measuring and control technology.
- Health and safety technology: To protect the operating personnel from the highly irritating ozone, appropriate health and safety technology is to be installed.
Figure 1 shows the integration of an ozone plant into the municipal wastewater treatment process.
-
> Advanced Oxidation Processes
Pulse CMS Advanced oxidation processes (AOPs) are based on the oxidation of the wastewater constituents caused by hydroxyl radicals (∙OH). The radicals are highly reactive. They react very quickly and unselectively with all organic compounds. They have to be produced on site in order to use them in wastewater treatment. The following process combinations are used for radical formation:
- Ozonation and addition of hydrogen peroxide (H2O2)
- Ozone and irradiation with ultraviolet light (UV)
- Irradiation with ultraviolet light (UV) and addition of hydrogen peroxide (H2O2)
- Irradiation with ultraviolet light (UV) and addition of titanium (IV) oxide (TiO2)
- Fenton's reagent (Fe2 + and H2O2)
AOPs are often used for the treatment of industrial wastewater, which often shows high concentrations of problematic substances. However, they are rarely used in municipal wastewater treatment. The recent findings are based on laboratory tests and isolated pilot projects.
In contrast to ozonation, AOPs show a higher micropollutant elimination rate. Even concentration of persistent substances, which are difficult to remove with ozone, can be reduced to a relatively high extent in wastewater. However, the efficiency of the processes depends on the effluent matrix, since the OH radicals also react with other wastewater constituents.
DWA Landesverband Baden-Württemberg
Rennstraße 8 | 70499 Stuttgart | Phone: 0711 89 66 31-0 | E-Mail: info(at)dwa-bw.de
© Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall e. V. // (DWA)
DWA Landesverband
Baden-Württemberg
Rennstraße 8 | 70499 Stuttgart
Phone: 0711 89 66 31-0
E-Mail: info(at)dwa-bw.de
© Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall e. V. // (DWA)

